Table of Contents
The Sun God Helios and Earth’s Rare Matter
In ancient Greece, the sun was called Helios and worshiped as a god. In ancient Greek mythology, the sun god Helios floated high in the sky, illuminating the entire world and looking down on everything on earth. According to the myth, Helios even witnessed Aphrodite, the goddess of beauty, cheating on her husband. The sun god reported what he saw to Aphrodite’s husband, Hephaestus, who, as the god of blacksmiths, built an invisible trap and placed it in Aphrodite’s bed. Aphrodite was caught in the act, and Hephaestus denounced his wife’s infidelity in front of all the gods of Olympus.
Years passed, and by the 19th century, European scientists were able to study the sun in more detail, and not just as a myth. Thanks to the development of a device called a spectrometer, they were able to precisely break down and measure the colors of light. Scientists took on the challenge of breaking down the sun’s light with a spectrometer to look at its colors, and what they found was a very strange mix of colors in the ubiquitous sunlight. The sun’s light is so bright and full of colors that it’s hard to tell exactly what colors are mixed together and in what amounts.
But with the help of spectroscopy, researchers found colors in the sun’s light that are hard to see on the ground. It was a subtle difference, but it was clearly different from the colors we see on Earth, such as the glow from burning something, the colors of different paints or pigments, or the colors that appear when objects are heated.
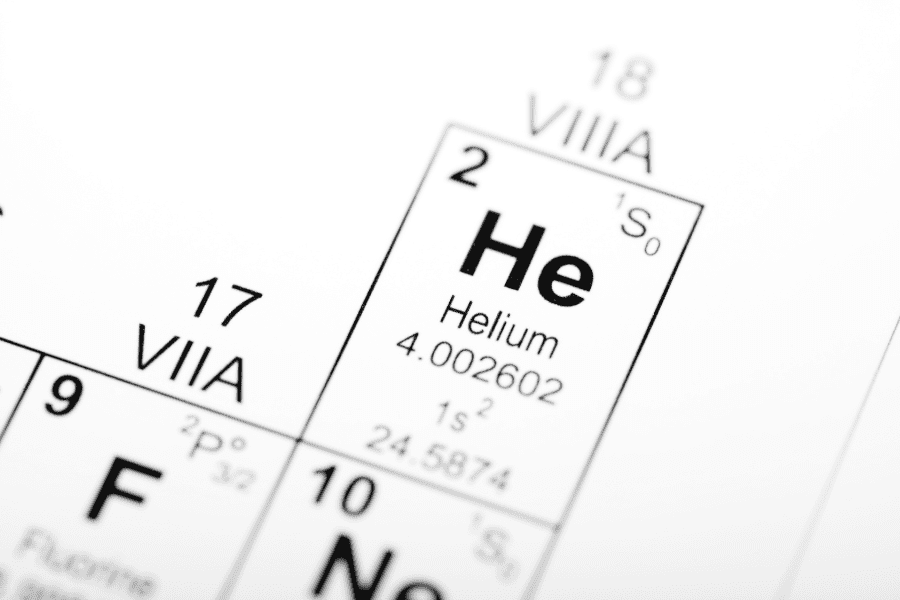
This led scientists to speculate that there was a substance on the Sun that was either absent or very rare on Earth, and that this substance was responsible for the unique color change in the Sun’s light. They decided to call the presumed substance in the Sun Helios, after the sun god Helios. This substance is helium, the second lightest atom in the universe after hydrogen. Modern research shows that there is actually only 0.0005% helium in Earth’s air, but the sun is about 25% helium by weight. The old scientists were right when they guessed that the sun would have a lot of a very rare substance, which means that helium is the stuff of the sun gods.
Nuclear Fusion in the Sun and Helium in the Universe
In fact, the Sun is a giant ball of hydrogen, with more than 70% of its weight in hydrogen. However, hydrogen atoms can merge into helium atoms in several steps at extremely high temperatures and pressures. This process is called nuclear fusion because the nuclei of the atoms stick together, and it produces intense light and heat. This is completely different from simply having two hydrogen atoms join together to form hydrogen gas. The hydrogen atoms stop being hydrogen atoms and turn into a completely different atom called helium. This phenomenon is so much more difficult than a normal chemical reaction that it’s not usually even considered a chemical reaction.
However, in a large, heavy, and hot sun, hydrogen undergoes nuclear fusion and turns into helium all the time. This is why the sun sends out intense light and heat in all directions, and why we live in a bright, warm world. On Earth, we can harness the power of nuclear weapons to artificially create fusion reactions, which are called hydrogen bombs. In a way, the sun shines because hydrogen bombs are constantly exploding.
The Sun is not the only star that produces light and heat from nuclear fusion reactions in this way. The Sun has been burning so hot and emitting so much heat and light for about 5 billion years. It’s still more than 25 percent helium and 70 percent hydrogen, so it will continue to burn brightly for a long time to come. In the meantime, it will have less and less hydrogen and more and more helium.
Helium on Earth and its Uses
But all the other stars in the universe are going through a similar process to the Sun, so if we were to summarize the changes in the universe in one word, we could describe it as less hydrogen and more helium over a very long time. So how much helium is there in the universe?
Studies have shown that right after the universe first formed in a big bang, there was a lot of helium in that primordial moment. In the entire universe, hydrogen is the most abundant element, followed by helium, and 90% of the atoms that make up the universe today are either hydrogen or helium. In other words, the atoms that make up all the landscapes, buildings, valuables, animals, plants, people, etc. on Earth, minus hydrogen and helium, make up only 10% of the universe.
Hydrogen and helium are abundant not only in the Sun, but also in the large planets of the solar system. Jupiter and Saturn have helium that makes up 5 to 10 percent of their weight, but there’s very little of it on Earth. This means that our planet is unique, strange, and rare. In the 19th century, scientists observing the sun’s light labeled helium as a substance from the sun that we don’t have on Earth, so it’s possible that we didn’t even realize it was so common in the universe.
Now that science has made it possible for us to get a little bit of what we thought was only found in the sun, helium has turned out to be quite useful and is used in many fields. In recent years, the price of helium has suddenly increased, making it unexpectedly expensive, and sometimes it seems like countries are competing with each other for it. With so many different uses for helium, it’s not surprising that we’re seeing more and more fights over it. This is why some people have even started investing in the price of helium to make money.
The reason why helium is so useful is not because it has an unusual chemical reaction. On the contrary, it’s useful because it doesn’t do any chemical reactions. It won’t burn when you put it on fire, and it won’t rust when you put metal in it. In short, helium is a substance whose most important property is that it has no properties.
The Dangers of Helium Balloons and Hydrogen in Amusement Parks

Substances like neon and argon, which are in the same column as helium on the periodic table, all tend to have very few chemical reactions. This is why they are collectively called noble gases, and helium is one of the most inert gases.
Helium is used in balloons that attract children at amusement parks. Hydrogen and helium are lighter than air, so when you add them to a balloon, it floats up into the sky. Children at amusement parks love to carry helium balloons because they’re fun and exciting. It’s not uncommon to see children at amusement parks who spot a colorful helium balloon and cry out to their parents, “I want one of those, too!” or who are delighted to hold the balloon in their hands, only to be disappointed when they accidentally miss it and it goes up into the sky.
The lightest and most common atom in the world is hydrogen. It’s also easy to get because we can make it in large quantities from other common substances like water. So why not put hydrogen gas in balloons? Because hydrogen gas is very reactive. If you were to light a match to a balloon filled with hydrogen gas, it would quickly burst into flames. This is a classic chemical reaction, and it’s dangerous to put something that can easily catch fire in a balloon for children to play with.
In the movie Indiana Jones And The Last Crusade (1989), there is an airship that flies through the sky suspended from a giant balloon, and in the 1930s, there were companies that operated giant airships using cheap hydrogen gas in balloons. However, in 1937, there was a major accident in which the hydrogen gas caught fire, as in the Hindenburg airship disaster. Shortly thereafter, hydrogen-powered airships were eventually abandoned due to the risk of fire.
Helium is not at risk of such accidents. It doesn’t catch fire from embers, and it doesn’t change at all when subjected to extreme temperatures and pressures. This makes helium balloons perfect for children to play with.
Helium Applications in Industry

However, helium is too scarce on Earth, being much more expensive and difficult to obtain than hydrogen. If helium were as common on our planet as it is in other parts of the universe, we would be able to take advantage of the abundance of helium in the world and play with balloons to our hearts’ content. And since you can make balloons as big as you want, you could probably float around in the sky, float all kinds of facilities, and develop a culture where people take flying in balloons for granted. But on Earth, helium is only obtained by digging up gas that is accidentally trapped in a fissure deep in the ground and extracting the helium from it. That’s why helium is so expensive.
People sending leaflets from South Korea to North Korea used to use helium to launch balloons, but it was too expensive, so now they use hydrogen gas in the balloons, and then suspend the leaflets in the sky. Nowadays, opponents of leafleting in North Korea point out that the use of hydrogen gas is a fire hazard.
Aside from its ability to catch the eye of children at amusement parks and make them beg for balloons, helium is also useful in industry. Of course, it’s important to note that helium has no properties, meaning that it doesn’t undergo any chemical reactions.
Imagine a workshop where you need to do very precise machining or apply high heat. If you need to make something precise and there are chemicals around that are very reactive, unnecessary chemical reactions can occur and damage the material. For example, in a semiconductor factory, where materials are shaved to a hundred-thousandth of a millimeter accuracy to make products, even the slightest rusting of the material from being exposed to ordinary air can ruin the product. Or if you’re making a product with a machine that applies intense heat or electricity, you’re worried that the high heat could cause the material to catch fire or start an unwanted chemical reaction. This is where blowing helium into a manufacturing facility comes in, as it fills the environment with helium and creates a clean environment with nothing to cause unwanted chemical reactions. Naturally, the materials won’t catch fire. In fact, semiconductor factories in South Korea buy helium and blow it into their manufacturing facilities.
Rocket engines and the role of helium
Similarly, helium is used in rocket engines. You might wonder how helium, which is neither flammable nor explosive, could be useful for launching a rocket, but depending on how the engine works, the components that use it can be crucial.
South Korea’s Nuriho rocket is designed to quickly detonate kerosene derived from petroleum and use the force of the explosion to propel it upward. These rockets need to continuously release fuel from their fuel canisters, and they need it to explode just where they need it. However, when launching a rocket, there is a risk that the force of the fuel explosion can backfire and cause the rocket body to catch fire. Not only will this prevent the rocket from flying properly, but it can also cause the entire fuel canister to catch fire and crush the rocket.
To prevent this from happening, the piping inside the rocket must be blocked by another strong force to keep the material flowing in the desired direction. The Nuriho rocket uses liquid helium to create such a device. Helium is normally a gas, but it can be turned into a liquid by cooling it very cold and squeezing it under high pressure. You can store helium at this high pressure, and then, when you need it, open the lid and push it hard enough to blow out the pipe. You can think of it as blowing helium with such force that it pushes other fumes out of the way. If you used anything other than helium to do this, the high pressure and temperature of the rocket fuel would cause a chemical reaction that would burn itself out. Helium is so non-chemical that you can blow it into the middle of a rocket’s fire and it won’t have any effect.
Cryogenics and liquid helium

More recently, liquid helium has been used to make things very cold. When a substance is liquid, it means that the particles that make up the substance are somewhat stuck together. This is why liquid substances form visible shapes. They form droplets, and when you put them in a cup, they clump together in the shape of the cup. Gaseous substances, on the other hand, are tiny particles, flying around separately.
However, helium is so non-chemical that it doesn’t react well with itself. It’s hard to make it into a liquid. Mercury, on the other hand, becomes liquid as soon as the temperature drops below 356.7 degrees Celsius, and the atoms stick together. No matter how hot it is, even when water is boiling, mercury always stays liquid and glistening in a cup. Helium, on the other hand, is always a gas, no matter what. No matter how much you lower the temperature, helium doesn’t stick together. To get helium atoms flying around at normal 1 atmosphere to stick together and become a liquid, the temperature would have to drop to minus 269 degrees Celsius. Theoretically, no other substance in the world can go below minus 273 degrees Celsius. This means that helium atoms can only get down to a temperature that is only 4 degrees Celsius above the lowest temperature limit imaginable before they stop scattering and flying around and start sticking together. Because it has to be cooled so dramatically to make it liquid, once it’s liquid, the ambient temperature of the helium can be made very low. What’s more, helium doesn’t react chemically, so you can use it for cooling without worrying about the effects on your materials.
If you’re only focused on making things cold, you can also turn more common substances like oxygen into liquids and use them for cooling. However, spraying oxygen all over something can cause it to react with it and change its properties. In particular, oxygen is a chemical reaction that can cause something to burn, so if you spray liquid oxygen on something to cool it down, it can catch fire.
Liquid nitrogen, which is more common and cheaper than helium, is also often used to make something very cold. However, liquid helium can be made even colder than liquid nitrogen, so if you need particularly low temperatures, you’ll have to spend a lot more money on it. This makes helium a must-have for specialized equipment or experiments that require very low temperatures. Helium is especially common when you need to take advantage of the phenomenon of superconductivity, where electrical resistance disappears at very low temperatures.
Helium in MRI and Human Health
One of the most common uses of liquid helium these days is in magnetic resonance imaging, or MRI, which is used in hospitals to look inside the human body. To operate an MRI, magnets are used to create a strong magnetic field, which needs to be kept at a very low temperature. And to keep the temperature of the magnets in an MRI machine down, you need to fill the machine with liquid helium. Sometimes, the helium in the MRI machine leaks out by mistake, and because the helium temperature is so low, it fumes all over the place. If you don’t know what’s going on and you see it from a distance, you might think it’s smoke from a fire, and there’s a lot of commotion and people evacuate. If MRI is used to detect a tumor or cancer in someone’s body, treat it in time, and restore their health, we can say that helium also played a role in saving their life. This is purely a result of the advancement of science and technology, but we can also literally say that the breath of the sun god saved the person’s life.
The Helium Shortage and future of helium

MRI machines are becoming more and more common in hospitals. There are also more and more precision factories that need to use helium, such as semiconductor factories. Korea imports 1,000 to 2,000 tons of helium every year, so we hear more and more about the need to use helium sparingly. In the future, we may have to use helium even more conservatively than we do now, as the number of places where helium must be used will increase and the number of places where helium can be found will decrease. It is also possible that we will no longer see helium balloons in amusement parks for fun. If you see a child playing with helium balloons in an amusement park, you may have seen a special scene that can only be seen in this era. If that’s the case, while you’re at it, you might want to consider the fact that helium is so precious that it contains the breath of the sun god Helios.
Discover More
If this article made you happy and taught you something, I’m sure our next article will do the same for you. Each piece is carefully written to help you understand things better and enjoy reading more. So, to keep exploring and having fun, just click on this link to go to our next story.